Search Dictionary
Version history
- Current: Jan 24, 2024
- Jan 21, 2022
Acid
There are at least three well-accepted definitions of “acid” and “base”:
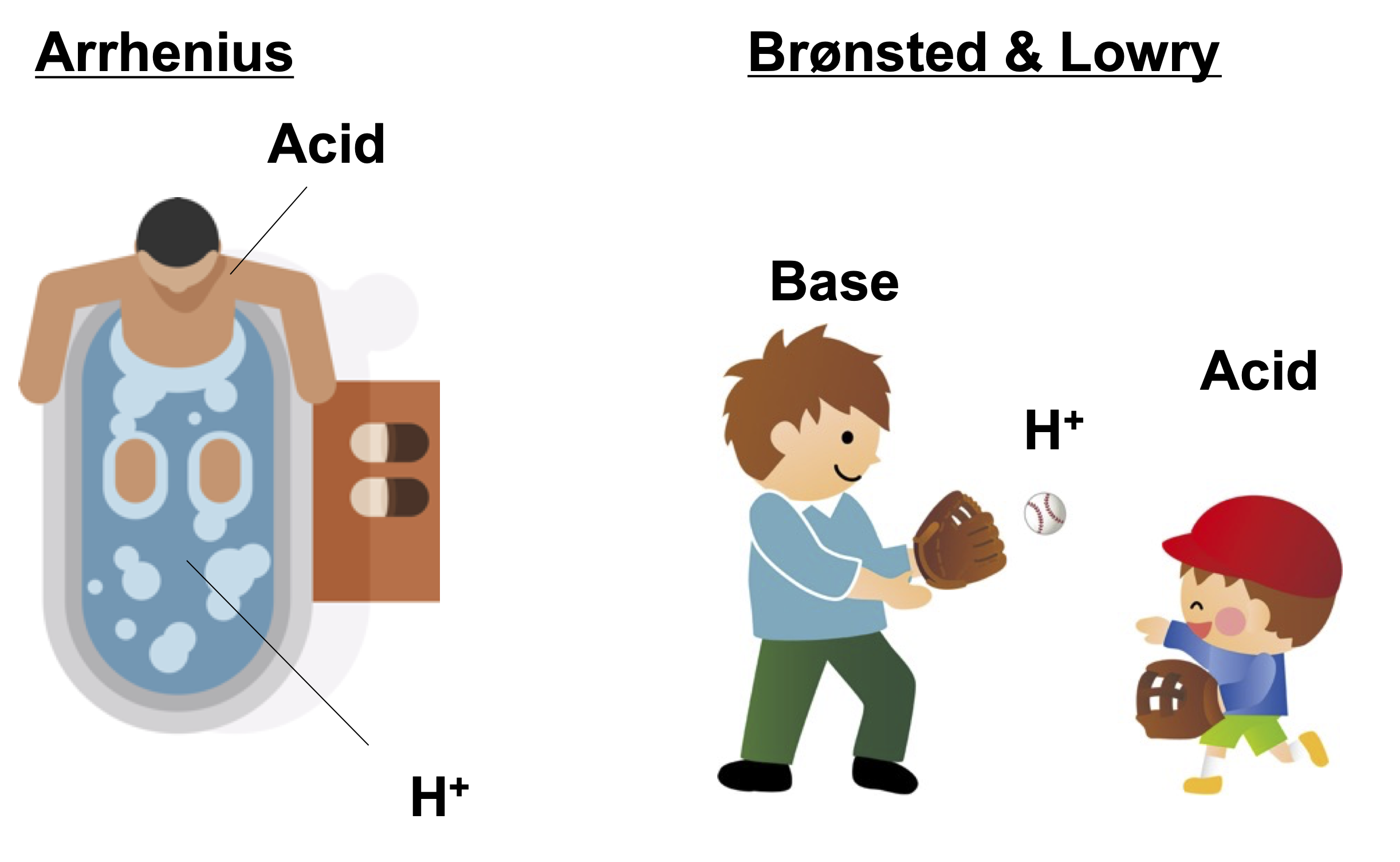
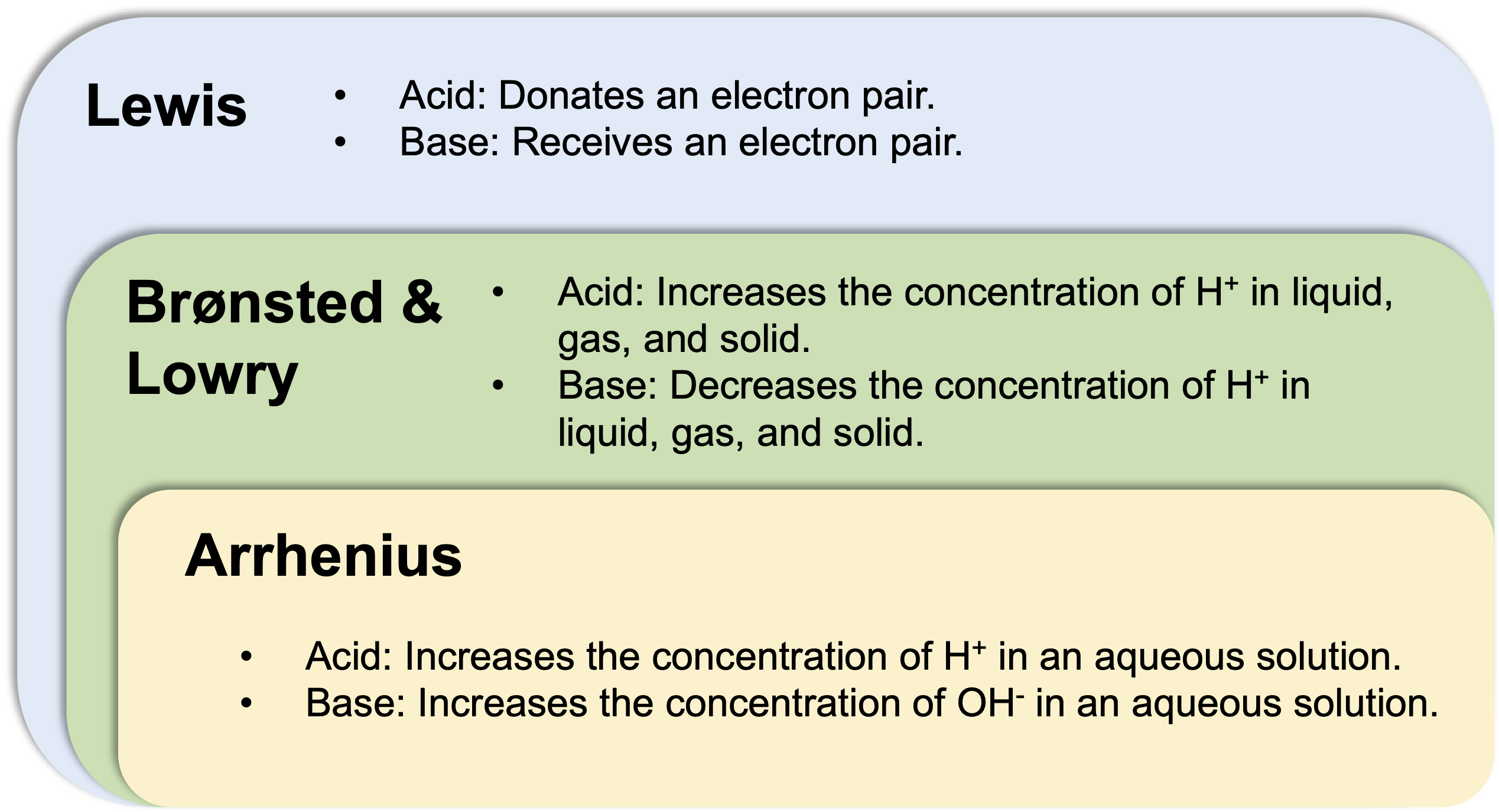
- Arrhenius definition: An acid is a substance that increases the concentration of H+ in an aqueous solution.
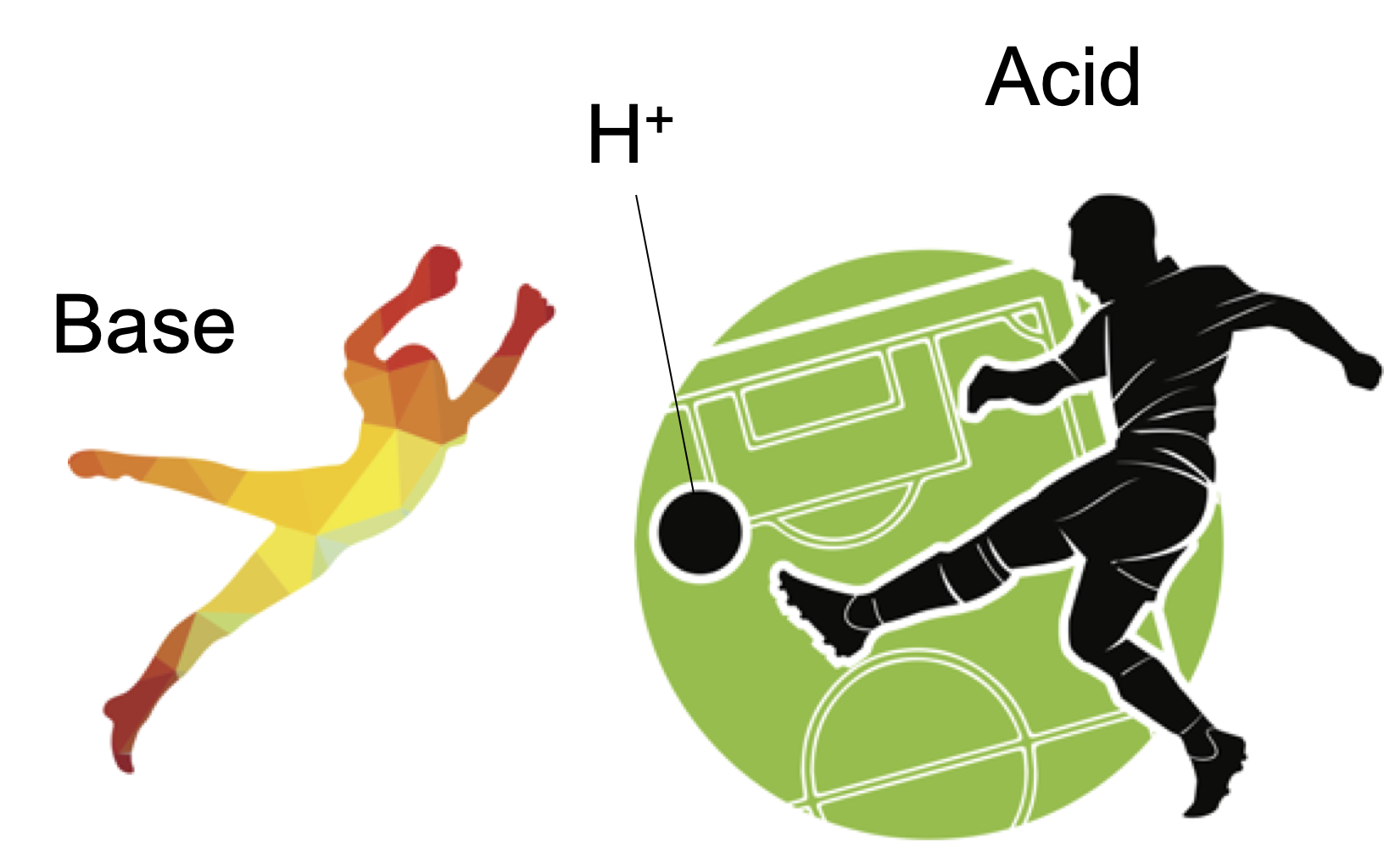
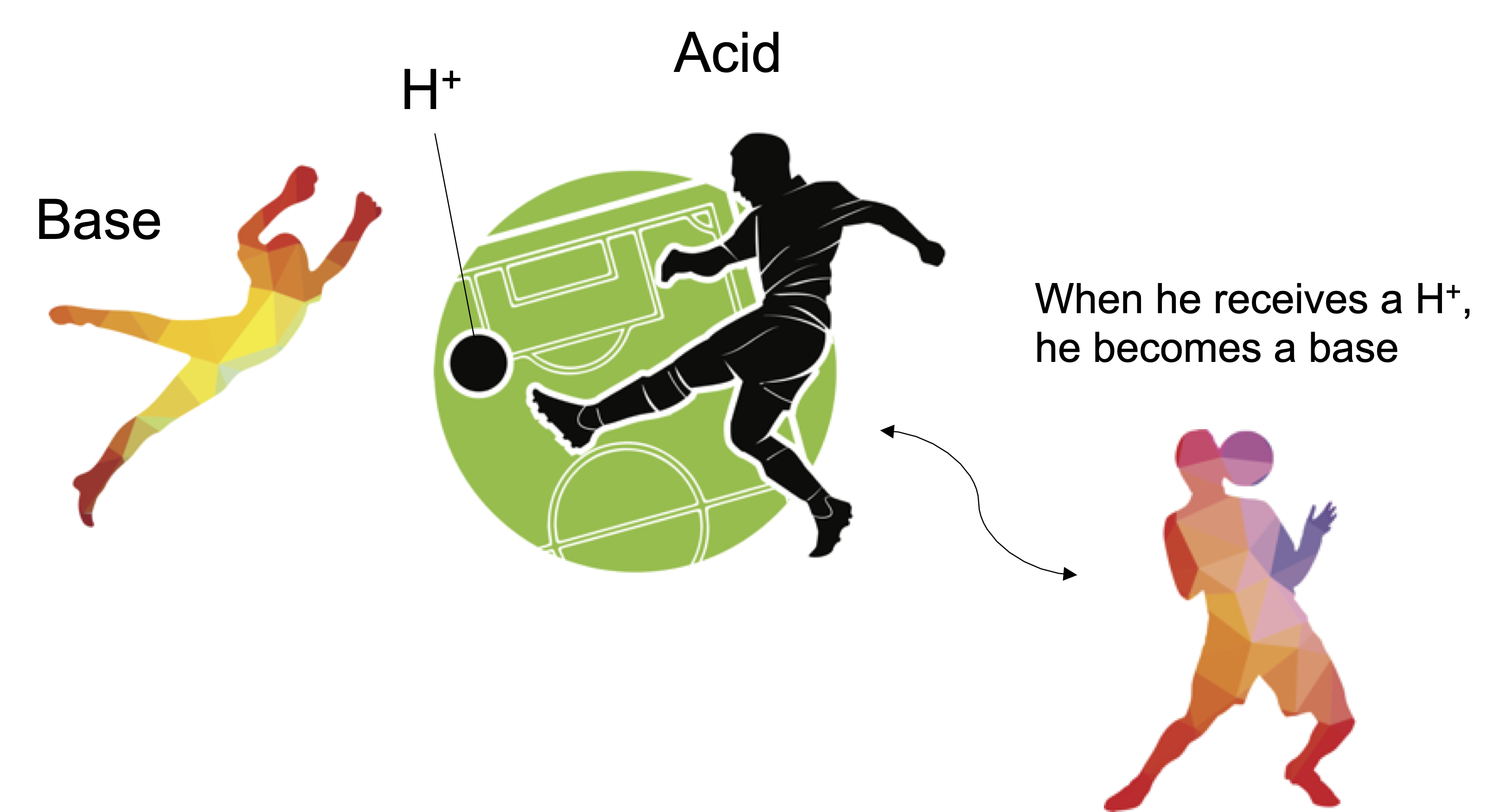
- Brønsted & Lowry definition: An acid is a substance that increases the concentration H+ in liquid, gas, and solid.
- Lewis definition: An acid is a substance that donates an electron pair.
The Arrhenius acid concept is valid only in an aqueous solution. The Brønsted & Lowry definition is the generalization of the Arrhenius acid to cover the aprotic solvents. The Lewis definition further expands the concept of acid and base to conver reactions that do not involve H+.
The strength of an acid is expressed as pKa, the negative log of acid dissociation constant. The stronger the acid, the lower is its pKa value.
Definitions in the literature
- a substance that behaves as a proton (H+) donor [1].
Also see:
Images
Click to see full-size images. All images are Public Domain.