Search Dictionary
Version history
- Current: Jan 21, 2022
Base
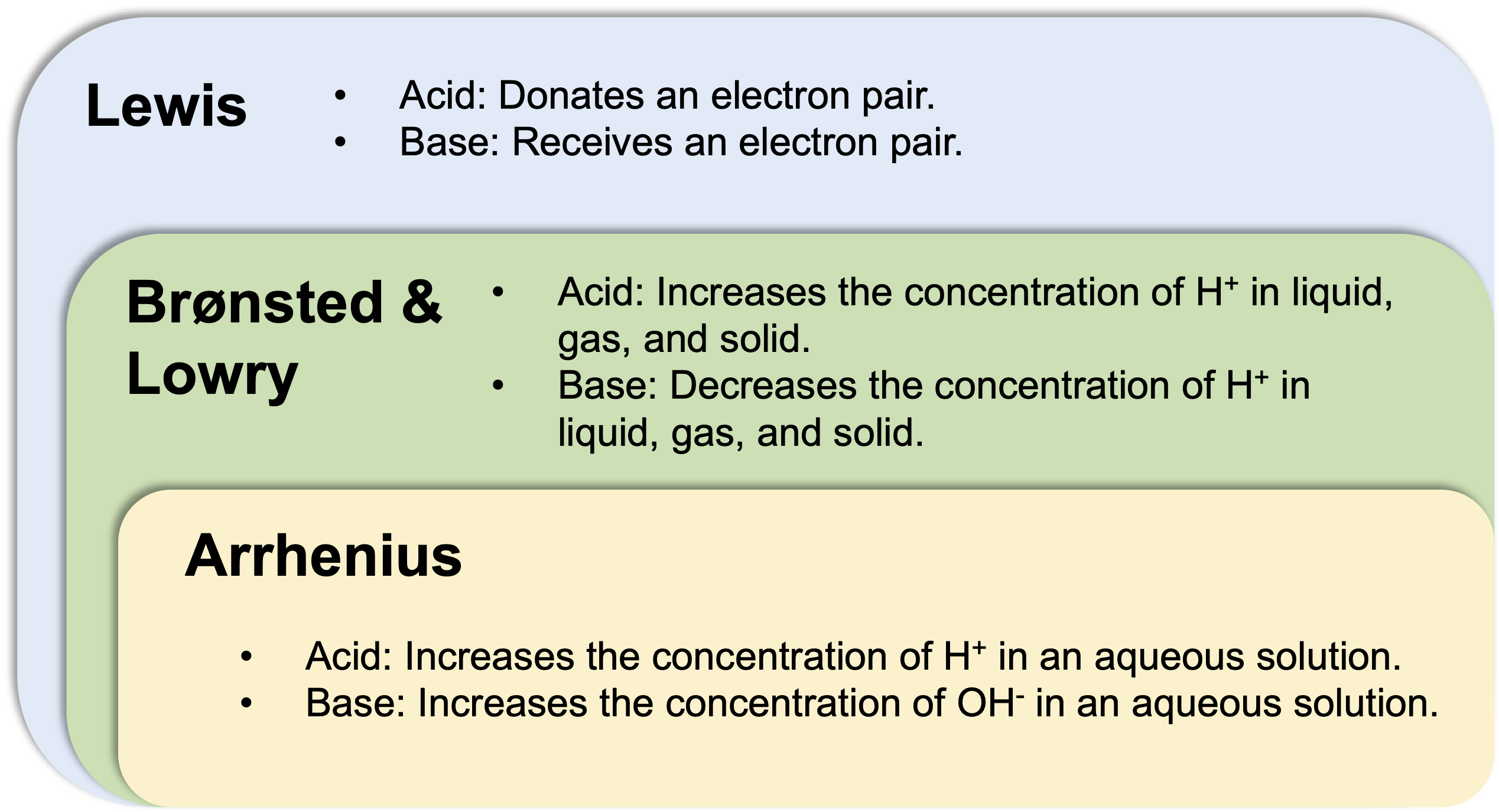
There are at least three well-accepted definitions of “acid” and “base”:
- Arrhenius definition: A base is a substance that increases the concentration of OH- in an aqueous solution.
- Brønsted & Lowry definition: A base is a substance that decreases the concentration H+ in liquid, gas, and solid.
- Lewis definition: A base is a substance that accepts an electron pair.
The Arrhenius base concept is valid only in an aqueous solution. The Brønsted & Lowry definition is the generalization of the Arrhenius base to cover the aprotic solvents. The Lewis definition further expands the concept of acid and base to conver reactions that do not involve H+.
Definitions in the literature
- a substance that behaves as a proton (H+) acceptor [1].

