Search Dictionary
Version history
- Current: Jan 14, 2023
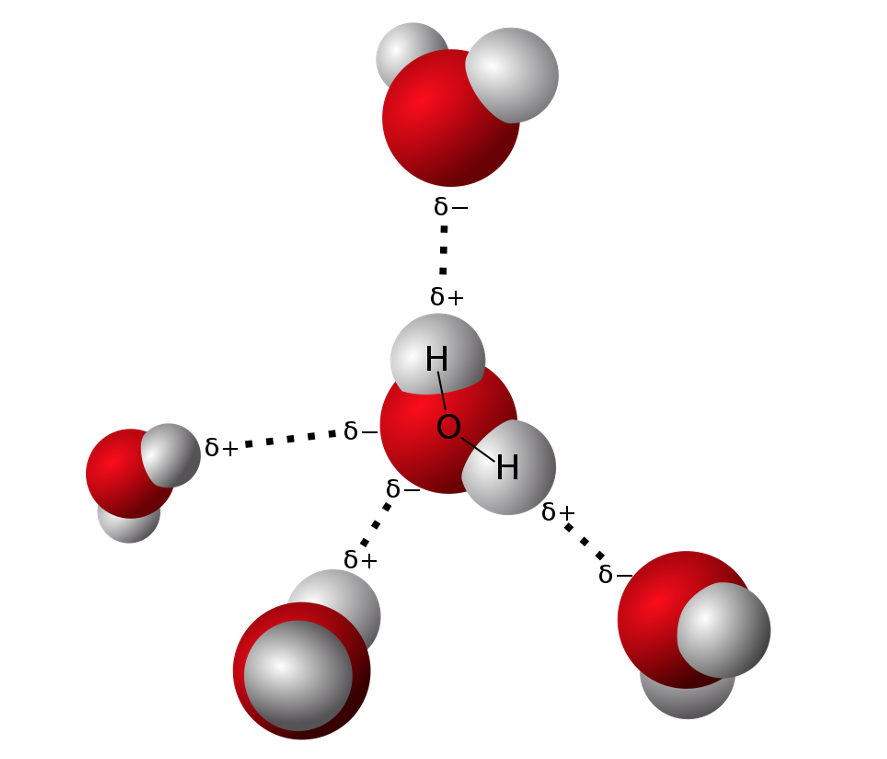
Hydrogen bond
A hydrogen bond is electrostatic attraction between a hydrogen atom covalently bonded to an electronegative atom (typically oxygen, nitrogen, or fluorine) and another electronegative atom.
Hydrogen bond can be formed between such atoms in a single molecule or of different molecules.
Definitions in the literature
- Attraction between a covalently bonded hydrogen atom and another atom taking part in a separate covalent bond [1].
- the weak attraction between a hydrogen atom that bears a partial positive charge (due to polar covalent bonding with another atom) and another atom (oxygen, nitrogen, or fluorine) that bears a partial negative charge; hydrogen bonds may form between atoms of a single molecule or of different molecules [2].
- the attractive force between a hydrogen atom covalently bonded to a small, highly electronegative atom and another atom containing an unshared pair of electrons [3].
- A type of electrostatic interaction between electronegative (fluorine, nitrogen, or oxygen) atoms in one molecule and hydrogen atoms bound to electronegative atoms in another molecule [4].

